BIOLOGICALLY
STRUCTURED MATERIALS
Relly Victoria V. Petrescu
Bucharest Polytechnic University, Romania
E-mail: rvvpetrescu@gmail.com
Raffaella Aversa
Department of Architecture and Industrial Design,
Second University of Naples, Italy
E-mail: raffaella.aversa@unicampania.it
Antonio Apicella
Department of Architecture and Industrial Design,
Second University of Naples, Italy
E-mail: antonio.apicella@unicampania.it
Florian Ion Tiberiu Petrescu
IFToMM, Romania
E-mail: fitpetrescu@gmail.com
Submission: 25/06/2019
Accept: 26/06/2019
ABSTRACT
In this paper bio-tissue mathematical modeling serves as a central
repository to interface
design, simulation, and tissue fabrication. Finite element
computer analyses will be used to study the role of local tissue
mechanics on endochondral ossification patterns, skeletal morphology and mandible
thickness distributions using single
and multi-phase continuum
material representations of clinical cases of patients implanted with the traditional protocols. New protocols will be hypothesized for the use of the new biologically techno-structured hybrid materials.
Keywords: Biologically Structured Materials; Techno-structured materials Hybrid materials; Biotechnology; Bioengineering; Biomaterials, Bioactive scaffolds, Biomimetics, Endochondral ossification patterns; Finite Element
Analysis, Osteointegration, Skeletal
morphology, Tissue mechanics
1. INTRODUCTION
Biomimetics, biomechanics, and tissue engineering are three multidisciplinary fields that have been contemplated in this research to attain the objective
of improving prosthetic
implants reliability. Since testing and mathematical methods are closely
interlaced, a promising approach seemed to be the combination of in vitro and in vivo experiments with computer
simulations (in silico).
An innovative biomimetics and biomechanics approach, and a new synthetic structure providing a microenvironment, which is mechanically coherent and nutrient
conducive for tissue
osteoblast cell cultures used in regenerative medicine, are presented.
The novel hybrid ceramic-polymeric nanocomposites are mutually investigated by finite element analysis (FEA) biomimetic modeling, anatomic reconstruction, quantitative-computed-tomography characterization, computer design of tissue scaffold. The starting base materials are a class of innovative highly bioactive hybrid ceramic-polymeric materials set-up
by the proponent research
group that will be used as a bioactive matrix
for the preparation of in situ bio-mineralized techno- structured porous
nanocomposites.
This study treats biomimetics, biomechanics and tissue engineering as strongly
correlated multidisciplinary fields
combined to design
bone tissue scaffolds. The growth,
maintenance, and ossification of bone are fundamental and are regulated
by the mechanical cues that are imposed by physical
activities: this biomimetic/biomechanical approach will be pursued
in designing the experimental procedures for in vitro scaffold
mineralization and ossification. Bio-tissue mathematical modeling serves as a central
repository to interface
design, simulation, and tissue fabrication.
Finite element computer analyses will be used to study the role of local tissue
mechanics on endochondral ossification patterns, skeletal morphology and mandible
thickness distributions using single and multi-phase continuum material representations of clinical cases
of patients implanted with the traditional protocols. New protocols will be hypothesized for the use of the new biologically techno- structured hybrid materials.
It is known that the field of interdisciplinary research
of materials for biomedical applications is structured and calibrated today in the bone repair study, which is basically its basis.
The bones are considered to be a biological hybrid material
composed of an organic
component, collagen, and an inorganic nanocrystalline hydroxyapatite component. Both phases integrate on a nanoscale so that morphological and physical variables such as crystal
size, nano-orientation, short-order order
between the two components determine the characteristics of its nanostructure and therefore the mechanical properties of the different
types of bone Frost, 1964, 1990,
2004).
Based on bone regeneration criteria, new bioactive
biomaterials have been developed. These materials are expected
to favor the formation of bone tissue by stimulating the proliferation and differentiation of osteoblasts (SCHIRALDI et al., 2004; AVERSA
et al., 2016a; AVERSA
et al., 2016b; AVERSA
et al., 2016c; AVERSA
et al., 2016d; AVERSA
et al., 2017a; AVERSA et al., 2017b, 2019; SYED et
al., 2017).
The use of nanostructured materials similar to natural bone tissue is one of the most
promising options for bone healing.
Nanotechnologies for the implementation
of
organo-inorganic hybrid materials offer excellent chances to improve
the performance of existing
conventional bone implants
(AVERSA et al., 2016a; AVERSA
et al., 2017).
Current research evaluates the evolution of polymer-polymer hybrids for bone tissue repair, as well as chemical procedures that control
the nanostructuring of materials.
1.1.
The
objective of the paper is inherent in the following scientific fields;
· biomechanics and human biophysical
bone,
· Biomimetics: nanotechnology in
medicine for materials inspired by nature
·
Bioactive
scaffolds favoring osteointegration in nanocomposite and hybrid porous
structural matrices
1.2.
Biofidelity
advances
Recent studies of the mandible
(SCHWARTZ-DABNEY; DECHOW 2003, APICELLA et al., 2010; AVERSA et al., 2016a; AVERSA et al., 2016b) and FEM modeling
of teeth (SORRENTINO et al., 2007,
APICELLA et al., 2015).
Knowing the mechanical and adaptive features of the bone is an
essential issue in designing
new biomimetic prostheses to replace
a bone with minimal biological and biomechanical
invasiveness.
Biomimetics is the science
that investigates such aspects
and can be considered the natural junction between biology
and engineering. This competence convergence allows
the development of biological principles and models to produce
bio-inspired materials that can be used
to
fully design prosthetic tissues
and systems.
New generations of concepts could be generated by the
conscious investigation of biomimetics, which can provide clinical tools to restore the structural, biomechanical and aesthetic integrity of bone functions.
Recent advances in cellular
and molecular biology and material
engineering science
(nanotechnology) have established that biomimetics and tissue engineering are developed
to improve the integral integration of prosthetic and restorative implants (AVERSA et al., 2016a; AVERSA et al., 2016b; AVERSA et
al., 2016c; AVERSA et
al., 2016d; PERILLO
et al., 2010; ANNUNZIATA et al.,
2010).
Since last century,
parts of our body have been replaced
with artificial prostheses. The materials used for these devices
were chosen not to produce
adverse responses
in contact with human body tissues and physiological fluids.
The criteria for choosing a specific
biomaterial were related
to its biocompatibility and functionality, which could be directly
associated with interfacial bone/implant interactions at a nanometric level. Only in the 1990s, the study of these interference effects
was improved by the use
of thin nanometer
layers and surface changes.
It then generated
a great commercial interest in the orthopedic market of adopting new modified implants of nanoparticles
that promote soft and soft tissue
engineering.
1.3.
New
classes of Biomaterials
There are several ways in which living tissues
can react to synthetic implant materials,
but are essentially limited to their response
to the
interface material
Three main terms could describe
the behavior of biomaterials defined by Jones et al (2012),
Hutmacher (2000) and Hoppe (2011).
Namely,
tissue responses are divided
into:
• Bioinert
• Bioresorbable
• Bioactive
A further
classification of ceramic biomaterials can be made according to their reactivity to physiological fluids;
• bioinert, such as
Alumina for dental application;
• bioactive, such as
hydroxyapatite used as a coating on metallic
implants,
• The
active surface, such
as bio-glass or A-W bottles,
• bio-resorbed, such as tri-calcium phosphate
Further improvement of these properties can be accomplished using nanostructured
bioceramics that can be used as interactive
materials, helping natural
tissues heal by promoting tissue
regeneration and restoring physiological functions (SCHIRALDI et al 2004; MANO et al., 2004; MORALES-HERNANDEZ et al., 2012; MOURIÑO et al.,
2012).
This approach has been studied in this study to develop a new generation of nanostructured bio-ceramic-polymeric hybrids that can be used in a wider range of medical applications (PETRESCU; CALAUTIT, 2016a;
PETRESCU; CALAUTIT,
2016b; PETRESCU et al., 2015; PETRESCU et al., 2016a; PETRESCU et al., 2016b; PETRESCU
et al., 2016c; PETRESCU
et al., 2016d; PETRESCU et al.,
2017; PETRESCU et al., 2018).
Porosity is one of the keys to the success
of these materials and is increasingly adopted when
a narrowing of the bones is required
and strong implant stability.
1.4.
Tissue
engineering new perspectives
For several years, tissue engineering has benefited
from the combined
use of live stem cell intake in three-dimensional ceramic scaffolds.
This strategy
is completed to provide healthy cells directly to the damaged site (BONFIELD
et al., 1981; HENCH, 1993; HENCH, 2002; HENCH,
2010).
By
combining the traditional bio-ceramic implant with the already assimilated knowledge of stem cell growth and differentiation in clinically and productively developed osteogenic strategies.
Stem cells developed in ceramic nanobiocomposites should be adopted
in the case of extensive bone repair
with excellent prospects of good functional recovery and integration of
the hybrid
scaffold with bone.
Synthetic Hydroxyapatite (HAp) has been described
in the literature as an attractive material
for bone implants (KIM et al., 2004; MORALES-HERNANDEZ et al., 2012).
Since its adoption,
the most common and simplest
method of producing synthetic HAp is the solid reaction
between calcium and phosphate
ions, resulting in the formation
of powdered compounds which can be sintered
and recovered at elevated
temperature to form a compact polycrystalline structure
Julien 2007).
The HAp bioactivity is governed
by processing parameters, such as the initial
compounds, the size of the crystalline granules, their purity, and the ratio of calcium
to phosphorus atoms. In particular, nanocrystals have shown improved bioactivity due to their large surface area. The use of hydroxyapatite with nanoparticles has been proposed as a valid solution
for the consolidation of low strength
polymer scaffolds.
The use of HAp nanoparticles for new classes
of implants, biocompatible coatings, and high strength
nanocomposites can be developed (GORUSTOVICH et al 2010).
1.5.
Biomimetics
A
general feature
of several tough natural hybrid materials, such as bones, sea urine teeth, pearls, is the strong nanometric interaction between inorganic
and organic phases.
This feature allows the organic
phase to act as a nanometric scale as a plastic energy dissipation network that inhibits crack propagation (high resistance); s, in situ synthesis techniques, have been adopted to mimic naturally
occurring processes.
In particular, precipitation of hydroxyapatite (or other crystalline compounds) in a polymeric
matrix was considered a viable route to
produce biomimetic
composites.
1.6.
Organic-Inorganic
Hybrid Biomaterials
A
bioinspired material development approach considering the formation
of self- assembling hybrid organic-inorganic will favour the use of hybrids
in biomedical applications. The high versatility of these hybrids
offers main functional and structural advantages that lead to the possibility to tailor-design materials
in terms of shape,
and chemical and physical properties.
1.7.
Bioengineering
and Bioactive scaffolds
For nanotechnology nanomaterials, nanotechnology is increasingly adopted for emerging
applications such as coating systems or three-dimensional coating systems (tecto)
(AVERSA et al., 2016a; Karageorgiou et al., 2005; SORRENTINO et al., 2007). Critical,
micro and nanotechnologies show the potential to be used to produce
advanced models for fundamental studies, such as tissue engineering structures or bio-molecular
devices.
The
ideal material for bone
skeletons has always been a hot
topic for research. An ideal skeleton should provide a sufficiently rigid but durable
mesh to temporarily replace
the damaged bone. At the same time, it should be able to biodegrade after the formation
of new tissues and integrate
it fully (MONTHEARD et al., 1992; KABRA et al., 1991;
PELUSO et al., 1997; SCHIRALDI et al., 2004).
Amorphous nanoparticles were synthesized in the laboratory. A new class of polymer-ceramic hybrid materials simulating the mechanical behavior of the bone were used as potential candidates for scaffolding (SYED
et al., 2017).
The result of these self-assembled nanostructured composites was micro-foamed and tested as a new preimplantation skeleton that can accommodate osteoblast growth factors
or stem cells to differentiate osteoblasts.
1.8.
Biofidelity
models and FEM analysis
Understanding the biological mechanisms of healthy and healthy bone growth is an iterative process between
biology and engineering. During this process, knowing that reverse
engineering of a biological system can have positive feedback in the field of biology,
allowing for a more complete and secure understanding of the potential
path for further development
of applied medical
engineering.
The most important question is how clinician interference with biological systems
can be optimized to improve
treatment options
so as to increase treatment
efficiency and lead to a
more stable outcome.
The use of new diagnostic and engineering tools such as those used in our research
(for example, CT and CTM CT segmentation and solid CAD reconstruction) can detail the anatomy of soft and soft textures in a very precise manner as a small standard
deviation. Therefore, the integration of biological knowledge and clinical possibilities is essential. A more reliable and biophysical model begins with biomechanical
modeling of bones, ligaments
and alveolar bone,
using Finite Element
Analysis to gain insight into the biological response to changing biomechanical circumstances.
Because current tests and numerical methods
are closely related, a methodological approach is to combine in vitro and in vivo experiments with computer
simulations (in silico). There are, however, a number of stimulus
points involved in creating and realizing the mathematical
model. The concurrent interaction of several
variables that influence
the prosthetic system was investigated by simulation in the mathematical modeling of finite
elements.
Finite Element Analysis (FEA) involves the subdivision
of a
geometric model into a finite number of elements, each with specific
mechanical properties. The variables
to be investigated are guessed
with mathematical functions. Specific mathematical software evaluates the distribution of stresses and stresses in response
to changing charging conditions.
A complete
assessment of the mechanical behavior of a solid or prominent
biological structure
can be made, even in the case of non-homogeneous organisms. When properly validated by in vivo or in vitro testing, finite element
analysis is useful in defining
optimal recovery criteria and material
selection criteria
while allowing for potential
fracture prognosis in limited circumstances.
2. METHODS AND MATERIALS
2.1.
Materials
The hydrophilic matrix for amorphous silica amorphous filler
(Aerosil 300 Degussa, Germany) of 7 nm with a specific
surface area of 2 n-hydroxyethyl methacrylates (HEMA), Sigma-Aldrich Chemicals Co., was used. 300 m2g-1.
The initiator of the radical polymerization reactions used as azoisobutyronitrile (AIBN) obtained
from Fluka (Milan, Italy). HEMA monomers were mixed
with 10% by volume of silica.
The degassed resin was poured
into 2.5 mm thick flat molds and polymerized at room temperature, controlled
at 60 ° C for 24 hours. Another end of 1 hour at
90 ° C was finally made on
the nanocomposite plates.
Microporosity was induced in the dense material
by first balancing the samples in pure ethyl alcohol and then rapidly extracting the alcohol absorbed
by balancing into distilled water. During the rapid extraction of alcohol and counter-diffusion, a microporosity occurs in the ceramic-polymer hybrid material,
evidenced by the intense
bleaching of the treated system.
2.2.
Finite
Element Analysis (FEA)
Segmentation of medical images was derived from CT using the Mimics software
(Materialize, Belgium) to process a medical image to the patient.
As reported at the top of Figure 1, CT processing has led to a solid 3D solid model of anatomy and bone structure.
The combined use of Mimics and 3-Matic software (Materialize, Belgium) has been used
to obtain solid 3D models and Finite Element Analysis
(FEA).
External bone geometry was reconstituted by generating a three-dimensional volume that interpolates CT scans. The results were then imported
into the 3Matic
software for surface
and solids optimization, finite
element modeling and material properties of different sections
of the bone according to the characteristics of the literature (BEAUPRE; HAYES, 1985; REILLY; BURNSTAIN, 1974; REILLY; BURNSTAIN, 1975; HUISKES et al., 1987; SCHWARTZ et
al.; DECHOW, 2003; TÖYRÄSA
et al., 2001).
Bone stress can be modulated by choosing skeletal
swollen thickness for healthy
bone growth. In vivo tests performed using these
modified oral implants
confirmed the improved ability of these implants
to promote early osteointegration (GRAMAZZINI et al.,
2016).
Biomimetic/biomechanical
approach: Ceramic-polymer hybrid design
and
bulk design to improve osteointegration Bioprotective devices are widespread rehabilitation therapy in clinical
practice in many areas of rehabilitation surgery such as dentistry, maxillofacial surgery, orthopedics. The bone and implant
interface has been studied
for many years as we try to move from bioinert to bioactive
biomaterials.
In fact, the histological analysis carried out in these years does not allow confirmation of possible
contact theories, junction systems, and others. But there is fluid contact between the osteocyte channel and the implant surface. Bioactive biomaterials could favor and amplify
differentiation from an osteoblastic phenotype that occurs during healing
of surgical wounds caused by the implant,
with better osteointegration at shorter times. Recent studies
describe the characteristics of nanostructured materials that could
promote osteointegration:
·
Carbon and alumina
nanostructures, which mimic nano-dimensional geometry of hydroxyapatite, increase osteoblastic activity and thus produce larger bone deposits when
applied to orthopedic implants.
·
nanostructured biomaterials that mimic the bioactivity of hydroxyapatite crystals favor
the adhesion and production of
alkaline phosphates in osteoblast-like
cells
Therefore, further studies on these materials
could bring better and shorter
healing to promote
protocols to ensure early and immediate loading. The composition and the surface properties appear to be important
because they seem to modulate the response of the osteoblast cells affecting tissue healing
(DAVIS et al. 1991;
GRAMAZZINI et al., 2016; AVERSA, 2016b).
The implanted tissue adjusts its composition and architecture to its functional load (APICELLA et al., 2011; APICELLA et al., 2015). Therefore, a key to the success of the titanium implant to integrate
into the bone appears
to be whether bone remodeling is correct or not on the periphery of the implant
(AVERSA et al., 2016b).
Figure 1 shows the result of "in
vivo" experiments performed on dental implants
placed in rabbit white femur. In particular, the experiment described in Aversa et al. (2016b)
consisted of assessing the osteoinductivity and osteoconductivity of the Ti implant surfaces
without a 100-microns thin-layer ceramic-polymer hybrid material.
Bone implantation or bone thickening (COMERON, 1986), which is defined
as the percentage of bone implants for biomimetically implanted implants and not covered
by the six-month in vivo test, shows a significant improvement in approximately 100% growth over
two months, and 30% after 6
months.
The reconstruction of the bone micro-CT
bone around the implant
was validated using the physiological distributions of the calculated FEA strains. Maps of the maps surrounding the bone around the implant confirmed the critical role of the Ti-Bone
bioactive interface.
Osteoblast proliferation and bone growth in the implanted rabbit femur are clearly
favored and accelerated
by the presence of the
hybrid nanostructure layer.
The biomechanical approach utilizing adaptive bone properties describes the biomimetic behavior of the proposed
preimplantation hybrid scaffold as it can predict
bone resorption surfaces (elements of the FEA model with strains
below the lower physiological boundaries were deleted in the image) as shown in the in vivo implant
micro reconstruction microtubule on the right
side of Figure 1).
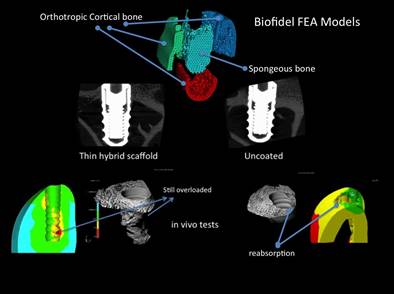
Figure 1: in silico and in vivo validation for
Osteoconduction of Titanium implants coated with a nanostructured hybrid osteoactive (left side) and without (right).
Research has shown that mechanical stimulation can have a profound effect on the differentiation and development of mesenchymal tissues.
Figure 2 illustrates the adaptive properties and strain threshold
values for healthy
bone growth.

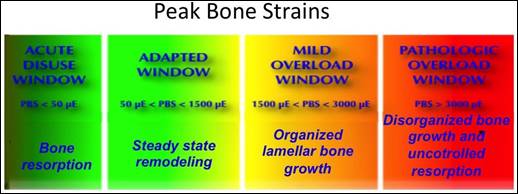
Figure 2: Adaptive window of bone physiology:
Structural adaptations to mechanical usage
Source: Frost (1990)
According to Frost (1990),
which quantified Wolff (1892)
above (> 3000 micro epsilon) and below (<50 micro epsilon)
of critical strain levels,
bone growth is impaired. In the mild strain region, healthy bone growth and regeneration is favored.
In fact, in order to maintain implant stability during pregnancy, it is of major importance for bone-forming osteoblasts to promote an extracellular matrix in the immediate vicinity of the implant.
Osteointegration mechanisms to be considered in biofidel models
Osteointegration of implants is essential for the rehabilitation of the prosthesis. Achieving and maintaining stable functional anchylosis has the following morpho- structural characteristics, namely:
·
direct contact between bone
and implant in the absence of the idoneous tissue interface;
·
the existence of the primary bone
in contact with the surface of the biomaterial;
·
deposition, externally to the
primary bone, of the lamellar secondary bone in contact with the titanium
surface;
·
increase in preimplantation
bone density compared to the normal bone region of the region;
·
Increase in medullary spaces,
which is required to evacuate the metabolic requirements of less involved
tissues in the region in the dissipation of the load;
·
Compact bone condensation,
which may be related to the load propagation patterns determined by the specific
morphology of the implant;
·
organizing a strong trabecular
structure that is radially removed from the compact preimplantation bone;
·
the presence of a bone crystal
wall at the level of the subepithelial conjunctiva, which may allow junctional tropism
and the formation of the liquid epithelium.
The mature matrix, which
has been described to occur in dental and orthopedic clinical trials,
is expected to provide mechanical stability of the implant even in the early osseointegration phase (primary stability). In fact, due to the hydrophilic nature of the hybrid material, high fluid levels are absorbed from the external fluid medium,
resulting in significant swelling and an initial increase
in the volume of hybrid glass material (Figure
3).
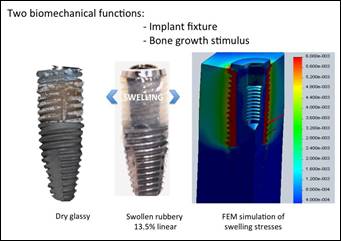
Figure 3: Mechanisms
of primary stability
and osteoinduction improvements
in Hybrid swellable scaffold modified Titanium implant. Glassy dry
scaffold (left)
The biomechanical and biomimetic active scheme has two biomechanical functions, the first being strictly related
to stabilization of the prosthetic system after implantation (the prosthesis can be loaded one hour after implantation), while the second function
is associated with stimulation of bone growth exerted by implanting the bone surface from
around her.
The volumetric expansion of the scaffolding can be effective in improving
the stability of the primary implant,
confirming the high bioactivity of the nanocomposite material tested (Figure 4), (PETRESCU; PETRESCU, 2019;
PETRESCU, 2019).
The presence of the inflatable implant component, which is supported by the upper part of the Ti core of the implant, increases the removal torque after
implantation when the system is in the
presence of organic fluids.
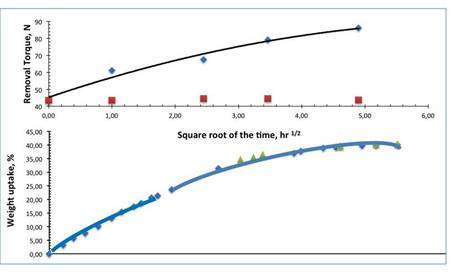
Figure 4: Physiological fluid uptake (bottom) and
implant stability improvement (top) of Hybrid swellable scaffold modified
Titanium implant.
The removal time, measured at different times after implantation, actually increased by more than 100% to 24. In addition,
even after one hour, the removal
torque already increased
from 43 to 62 N (an improvement of approximately 25%). It has been described in a previous
paper (AVERSA et al., 2016b) that improved retention has immediately followed the spinning kinetics of the hybrid material scaffold
(bottom of
Figure 4).
This increase in implant
stability is due to the strong compression strains generated in the swollen rubber hybrid scaffold,
as can be deduced from the map of the stained strains reported on the right side of Figure 3 (the red color of the implant hybrid insert).
The implant is then constrained in its socket by the external bone, which then increases
the retention and stability
of the implant.
Applying a larger
removal torque for explants
is, therefore, necessary
to increase the experimentally measured inflation
rate.
Furthermore, the constraint bone is subject to expansion strains as indicated by the colored map of the Von Mises strains reported
in Figure 3. The color scale used for this map is the same as that reported
in Figure 2, the physiology in which healthy growth and induction
correspond to colors of yellow and green and blue and red to bone
reabsorption.
The surrounding bones are subjected to healthy
bone deformation at a distance equivalent to the diameter of the implant. In this toroidal volume surrounding the implant,
an osteoinductive effect and a faster osteointegration of the implant
are expected.
A micro-computer
tomography confirmed these expectations.
Figure 5 shows the
micro CT of these volumes.
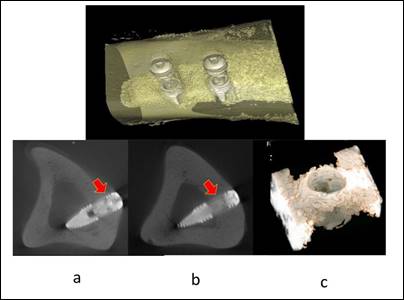
Figure 5: The Bone to Implant Contact (BIC) and the
relative bone density have shown similar characteristics at cortical (a) and
medullar levels (b), Bone near to the implants shows similar characteristics
(c)
Souce: Gramanzini
et al. (2016)
The upper part of the figure
reports bone reconstruction and external implants, while the lower part shows the 3D reconstruction of the volume surrounding an implant.
BIC and relative bone density had similar characteristics at cortical
(a) and medullary
(b) levels indicating good osteointegration of the initial bone implant.
The newly formed bone near the implanted implants has similar characteristics to the preceding (c), indicating the biomechanical stimulation effect of the swollen
and swollen hybrid material.
Traditional bone prostheses are mainly made of metals and ceramics
with remarkable strength
and stiffness, but with
high physiological invasiveness.
These implants, which are expected to serve for a longer
period of time, without failure
or surgical revision, although they guarantee mechanical and functional wood, occur frequently physiologically and mechanically with the human bone. Failures and lack of long-term reliability result
from incomplete integration of the personal characteristics
of the
bones and the patient
with local physiology.
To
reduce implant invasiveness, a biomimetic approach is suggested; the implant is expected
to have an "equivalent stiffness" (the combination of the elastic modulus of the material
and the prosthetic shape) that matches that of a bone area where it is implanted.
The bone modulus varies in size from 4 to 30 GPa, depending on the bone type and the direction
of measurement (bone
orthotropy). Current implant implants are isotropic with higher rigidities than bone (for example, between 160 and 210 GPa for titanium and steel alloys) that greatly
alter the physiological distribution of bone stress.
This behavior prevents the transfer
of biomechanically modified
stress to the adjacent bone, resulting in bone resorption around the implant
and, consequently, weakening
of the implant (this effect is
called stress reduction).
This unwanted biomechanical invasiveness is due to the fact that the bones are functional and structural complex
entities composed
of less rigid,
open and dense and rigid materials that
are combined to
provide rigidity both in orthotropic
forces and in cortical bone forces. Equivalent to the three-dimensional properties of bone tissue should be truly necessary for implanted
prostheses to achieve complete integration into the host bone tissue
(AVERSA et al., 2019).
However, for the replacement and regeneration of soft tissues
and osteoconductive and powerful mechanisms, porous and ceramic
metallic biomaterials and polymeric systems and other metals have been proposed (TAYLOR et al.,
2007; PARFITT, 1983;; PARFITT,
1994; MARTIN et al.,
1998).
From a biomechanical point of view, the structure
must be rigid enough to support physiological tasks,
but should not drastically exceed the stiffness
of the replaced
bone to avoid stress shielding. Attaching the implant to the surface
or to the bone matrix should be
improved by alternatives to reduce stress protection.
Clinical efficacy and long-term
reliability of bone prostheses have been thoroughly investigated by analyzing
finite elements to clarify the causes of the new replacement of invasive restoration (GRAMANZINI et al., 2016; AVERSA et al., 2016a; AVERSA et al., 2016b).
Figure 6 summarizes the main methodological steps
used in these studies:
(a) biophysical bone for investigation, (b) highlighting and weighing
fractured bone macroanalysis, (c) stress of microanalyses and strains on the bone-implant interface. The peak values and regions
of adaptive bone properties are shown in Figure 6d where unused, healthy and overloaded regimens are reported
in Figure 6d (FROST, 1990).
Bone prosthesis interference was recognized at two magnitude
levels, namely a micro scale (bone-implant interface, Figure 6c) and a macro
scale (Figure 6b).
The first small scale (Figure 6c) explains
the biological and micro-mechanical interactions of the synthetic biomaterial with bone-forming cells and highly adaptive adaptive properties, while the second on a macro scale (Figure 6b) determines the complete biomechanical functionality of the implant
material and the ability to restore the distribution of the biologic stress state in the
prosthesis.
Osteoblasts under specific biochemical and mechanical stimuli mature
and turn into osteocytes that mineralize the bone. The activity of osteoclasts under conditions that were not stimulated mechanically after prostheses could induce bone reabsorption in this new state of
mechanical equilibrium.
Figure 6d shows the adaptive
bone properties that are in the coupling between bone
formation and bone
reabsorption.

(a) (b) (c)
(d)
Figure 6: Finite element analysis tools for
biomechanical and biomimetic investigation: (a) Biofidel models of the bone,
(b) Macro Finite element analysis of the implanted bone for definition of the
stresses and strains physiological modifications, (c) Micro Finite Element
Analysis at the bone-implant interface, (d) Strain limits for bone adaptive
properties.
Source:
Frost (1998)
This process refers to bone formation in which osteoclast reabsorption via osteoclasts and renewed osteoblast generations of precursors dynamically replace
dynamics (Figure 7).
The coupling can then be considered a complex mechanism of dynamic remodeling involving interactions of different types of cells and control
stimuli. Mechanical stimulation should include physiological levels of the strain (Figure
6d) between 50m and 3000m (APICELLA et al., 2011; APICELLA
et al., 2015; AVERSA et al., 2009). Over 50 years
of osteocyte activity predominate, resulting in bone resorption of between 1500 and 3000 m, a slight increase
in lamellar bone,
predominantly over 3000,
with uncontrolled bone
growth or resorption.
3. RESULTS AND DISCUSSION
In the case of bone, which is a structural biological material
that undergoes a mechanically induced continuous renewal (Figure 7), the remodeling process
is controlled by a dynamic balance involving osteoclasts (linking bone cells) and homeostatic
renewal osteoblasts.
Osteoblasts under specific biochemical and mechanical stimuli may actually mature and turn into osteocytes that mineralize the bone. Instead, osteoclastic activity under conditions that were not stimulated mechanically after the prosthesis (ie in the stress
release area) could induce bone reabsorption in this new state of bio-mechanical
balance (Figure 7).
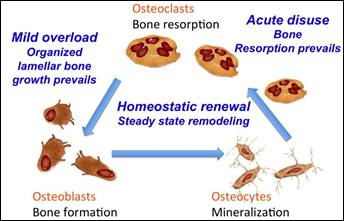
Figure
7:
Bone homeostasis mechanism
involving Osteoblast,
Osteocyte and Osteoclast
cells
Thus, in order to favor biomechanical integration and a longer maintenance period, a customized material, which carries
a great combination of high strength
and stiffness that fits the bone, must be used. The use of trabecular scaffolds and cortical bone to mimic behavior
and colon have been proposed
to recreate the distribution of macroscopic stress
and bone deformities but requires a necessary
micro-biomimetic interface that interacts with osteoblast osteoblasts.
Nanomaterials Ceramic-polymer nanoparticles based on hydrophilic and ceramic
polymers are potential candidates for new customizable biomaterials that will be used to cover porous phosphorus structures. The hybrid layer that comes into contact with bone can be customized by choosing nanophysics content and mechanical properties to achieve
the biomechanical characteristics necessary to increase
thickening of the osteoinductive bone in the porous
structure of the metallic
prosthesis.
These resulting hybrid systems
have the basic mechanical and biological properties that favor local healthy local generations. Specific and adjustable properties that simulate
bone tissue that acts on the interface guide microparticle, leading to bone marrow
growth and integration into host bone tissue. The main reasons for using the skeleton are therefore
to provide a bone formation environment, maintain space and support
the skeleton of the skeleton
during the repair process.
New
biomaterials that possess hybrid characteristics can be obtained by using nanometers in polymeric
matrices (AVERSA
et al., 2016a; AVERSA et al., 2017a;
AVERSA et al., 2017b), especially carbon nanofillers such as fullerenes and carbon nanotubes have been proposed to increase
strength and
stiffness nanocomposite materials. Although the use of carbon diamond
nanofilm can further
improve these
properties, some technological challenges
in production
technology should be overcome.
Graphite is only one of allotropic carbon forms and is thermodynamically stable at ambient temperatures and pressures, while the diamond
is another stable allotrope carbon
at high pressures
and at a temperature present in a metastable state at ambient
and similar conditions (PETRESCU; CALAUTIT,
2016 a-b).
Differences in the stability of allotropic forms are a consequence of the large energy barrier separating the graphite
Sp2 (left in figure 8) and diamond
sp3 configuration (even in figure 8), which requires
high temperatures and pressures in the presence
of the transformation catalyst.
However, an additional equilibrium parameter involving the surface
becomes critical and significant for the distribution of the equilibrium energy to the nano dimension: Gibbs free energy is drastically influenced by the presence of surface energy input, modifying
the phase diagram of the thermodynamic equilibrium (BADZIAG et al.,
2003; BARNARD et al., 2003; BARNARD; STERNBERG, 2007; VIECELLI
et al., 2001).
Atomic models (PETRESCU; PETRESCU, 2019; PETRESCU, 2019) have demonstrated that nanodiamonds with 3 nanometers with tetrahedral hydrocarbons are more stable than polyaromatic graphite under ambient conditions
(BADZIAG et al., 1990; AVERSA et
al., 2016a; AVERSA et
al., 2016b; AVERSA
et al., 2016c; AVERSA et
al., 2016d).
The presence of a more complex
structure, generated
at the nano-diamond interface, opens
up new interesting technology
applications. Cuboctodic structures
of 1.03 to 3.0 nm with onion structure characterized by the passage from Sp3 to Sp2 carbon
at their surfaces
were observed by Barnard and
Sternberg (2007).
A reversible reverse phase transformation in a nanodiamond-graphite cluster was observed
by Xiao et al. (2014)
appear in this morphological transition interface, which leads to daily bucky formation, characterized by a diamond core, a graphical case (schematized in Figure 9) (BARNARD;
STERNBERG, 2007). Such graphite
nano- diamond surfaces
can be modified
using graphite carbon chemistry to form cyclohexane functional systems such as Diels-Alder cycle reactions
between a conjugated diene and dienophiles (JARRE et al., 2011).
The nano-crystalline Sp2 and Sp3 Carbon structures open up a new perspective for future technological development in structural biomedical applications. The nanocrystalline particles produced by the detonation of carbon explosive materials (DANILENKO, 2004, GREINER
et al., 1988; OZAWA et al., 2007; CHANG et al., 2008) exhibit characteristic dimensions of 3-5 nm. Lubricants, galvanic coatings,
polymer nanoparticles, polishing systems, and niche applications recently used for electronics, emission devices, catalysts and combustion cells as nanocomposite membranes that lead protons
for the application of detonating nanodiamonds have been
proposed.
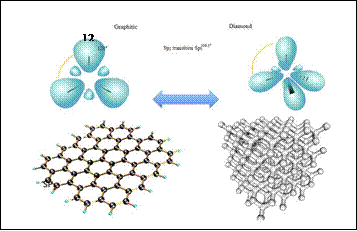
Figure
8: Left) Graphite (SP2 hybridization) and Right) Diamond (SP3 hybridization) Carbon
allotropic forms
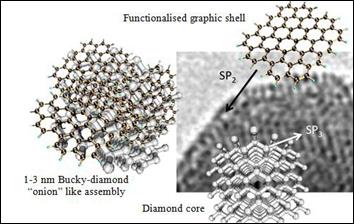
Figure
9: Bucky-Diamond “onion” like cluster (left side): Nano-diamond core (lower
right side) with external graphitic shell (upper right side) and TEM of a
detonation nano-diamond atomic structure (right side)
However, preliminary clinical and biochemical investigations have shown
that these
detonating nano-diamonds are biocompatible and non-toxic, opening up new biomedical applications, taking into account both the variety
of surface chemical
light and the intrinsic mechanical characteristics.
The detonation of nano-diamonds, characterized by different
levels of purity and the presence
of unwanted functional groups/elements, can not be
directly
appropriate for biomedical applications in which chemical purity and chemical
uniformity of the surface
(LAI; BARNARD, 2011a; LAI; BARNARD, 2011b). After raw production, these materials
are subjected to purification procedures.
A simple method uses surface oxidation and different levels of purity and surface
properties can be obtained.
Oxidation carried out at elevated temperatures in an air/ozone atmosphere can lead to a purification of the carbon fraction not present
as diamond up to 95% by weight
(OSSWALD et al., 2006; SHENDEROVA
et
al., 2011).
Oxidation of nano-diamond surfaces other than elimination of unwanted functional compounds forms oxygen-containing groups (the blue dots in Figures 8 and 9) such as
anhydrides and carboxylic acids (SHENDEROVA et al., 2011) which are suitable for the polarization
of hydrogen
or polar with the appropriate species.
By purifying
air/ozone, we can work on
the very reactive and hydrophilic surface of the carboxylate, which is very suitable for biomedical applications (KRUEGER et al., 2008; KRUEGER et al.,
2006).
However, the toxicity of nano-diamonds remains controversial and is a real concern. In vitro and in vivo analyses
are required to evaluate characteristics such as mechanical and physiological behaviors in vivo (SCHRAND et al., 2009a; SCHRAND
et al., 2009b; ZHANG et al., 2011; YUAN et al., 2010).
Although biocompatibility and negative effect have also been described
in the literature on the use of nano silica particles, our published
investigations have shown that nano-composite and hybrid materials
made by combining amorphous silica nanoparticles have reached a high level of biocompatibility,
and micro-nanoparticles
and
p-HEMA.
These
hydrogel hybrid nanocomposites
have been tested for
water sorption, water swelling,
and isotonic saline
and for fibroblasts and osteoblasts as a cellular response with adhesion, distribution and morphology tests.
The presence of polymer-bound silica makes these biomaterials excellent only for pHEMA.
Good properties of osteoinduction were also observed
for the differentiation of stem cells from the dental pulp Marrelli
et al. (2015).
These self-assembled nanostructured composites were also tested as a peri-implanter scheme to match osteoblast growth factors
or stem cells for osteoblast differentiation (MARRELLI et al., 2015). This effect was mainly determined by micro-mechanical stress and the voltage generated
at the bone/implant interface.
These new hybrid materials have been shown to be able to stimulate biomechanical bone growth within the range of physiological strains
that allow for healthy growth to allow for complete early and organized
integration of the implant into the receiving bone. Creating ideal scaffolds
for bones is a growing argument for research. Such an ideal
framework should provide a rigid and elastic mesh to temporarily replace
damaged bone function while creating a bioactive
substrate for bone regeneration, integrating it fully (MONTHEARD et al., 1992; KABRA et al., 2017;
AVERSA et al., 2017a;
AVERSA et al., 2017b).
4. PREPARATION OF MATERIALS
The 3 nm detonation nanoparticles (Aldrich, ≥97%) with a specific surface area of 400 m2g-1 were used as bioactive filling agents. Nanodiamond hydrophilization was performed (SHENDEROVA et al., 2011; AVERSA et al., 2016a; AVERSA et al., 2016b; AVERSA et al., 2016c; AVERSA et al., 2016d). The surface
graft decomposition followed by functionality consists of the aggregate
dispersion of ~ 20 nm. Treatment
of hydrogen at high temperatures leading to nanoparticles Nanoparticles Nanoparticle nanoparticles of 2-4 nm was isolated by centrifugal isolation at> 10,000 rpm (SHENDEROVA
et al., 2011).
The nanodispersion was mixed with 2-hydroxyethyl methacrylate monomers (HEMA) (Sigma-Aldrich Chemicals Co., St. Louis,
MO, USA). The suspension of HEMA nanomaterials (Figure 10) was radically polymerized in the presence
of the azoisobutyronitrile
thermal initiator (AIBN, Fluka Milano, Italy).
4.1.
The nanocarbon content was prepared
at 2 and 5% by volume.
The degassed reactive suspension was first transferred to 2.0 mm thick flat molds and then
polymerized at 60 ° C
for 24 hours. Finally,
a cure at 90 ° C was obtained.
4.2.
Finite
Element Analysis (FEA)
Solid models of dental implants were generated using the Solidwork 2016 software.
The titanium implant
and the hybrid material replacing one part were molded. The FE model was obtained by importing
solid models into ANSYS rel. 9.0 FEM software (Ansys Inc. Houston)
using the IGES format. The volumes were linked
to tetrahedral elements,
resulting in a 3D FE model consisting of 31,240 elements and 35,841 nodes. The precision of the model
was verified by the convergence
test.
4.3.
Mechanical
characterization
The effect of elasticity measurement on the dried, swollen
and pendulous p-HEMA nanostructure was performed using a DTM METTLER-TOLEDO mechanical shear modulator (Zurich, Switzerland). The elastic
and viscous components of the shear modulus were measured with a constant
frequency in an isothermal state. The samples were vacuum dried at 60 ° C for 24 hours before testing. In the shear test mode,
discs of 10 mm diameter and 2 mm thick
discs are placed between three steel plates forming a symmetrical sandwich. An isothermal scan was performed
at 37 ° C in a nitrogen purged medium. Deformation control was set at 10 μm and a force limit of 0.9
N was applied at an oscillating frequency of 10 Hz (AVERSA et al., 2019).
The presence of the oxidized functional detonated nanodiamond in the monomer reaction
mixture favors the self-orientation of HEMA polar monomers
(Figure 10). The nanoparticles are in fact characterized by the presence of oxygen-containing groups leading
to a preferred
orientation and self-assembly of the HEMA hydroxyl
group monomers on the
nanoparticle sizing surface (left side of Figure
10).

Figure
10: Self-assembly of HEMA monomers in presence of functionalised Detonation Nano-diamond
(left side) and hypothesized Nanodiamonds pHEMA self assembled hybrid nanocomposite
(right side)
Nanosilica hybrid Nanocomposites have been described in a previous paper (Aversa
et al., 2016a) to show the self-assembled analog behavior that led to the formation
of nanostructured hybrid materials. Similarly, functional nanopowder that does not contain oxygen binding
atoms (red in Figure
10) and functional HEMA functional
groups can produce
monomer.

Figure
11: Camparison of mechanical shear properties on amorphous nanosilica and
crystalline nano-diamonds-pHEMA hybrid nano-composite
The polymerization of these silicates from HEMA / silica gel leads to the formation
of a nanostructured hybrid material
exhibiting specific
and specific properties, such as improved
mechanical stiffness and biocompatibility (AVERSA et al., 2016a;
PETRESCU et al., 2016a;
PETRESCU et al., 2016b; PETRESCU
et al., 2016c).
Applying this model of nanostructure formation to the nanodiamond / HEMA suspension polymerization, a similar improvement in mechanical properties and biocompatibility is expected.
However, improved mechanical properties are expected
to be more relevant due
to the high and high shear force
(Azo technology).
The stiffness of the synthetic diamond may be up to 15 times higher than that of silicon,
i.e. from the shear modulus
of about 450 GPa Vs about 30 GPa (Azo). By acting
as a filler or hybrid formation, nano-diamond detonation could generate the mechanical behavior of similar nanoparticle hybrids (AVERSA et al., 2016a), the behavior
of pure variation
according to the volume fraction
of hybrid diamond nanoparticles
in the material
could be assessed.
As
described by Aversa
et al. (2016a) aims at balancing
PHEMA hybrid nano- silicas
in physiological solutions. Water molecules that bind to polymeric hydrophilic groups induce a significant plasticization of the nanocomposite (in our case, a 16% by volume nanocomposite) that reduces the shear modulus of the dried initial samples from 8-9 GPa to
0.01- 1, 1 GPa 6, (AVERSA
et al., 2016d; AVERSA et al., 2019).
The compositional dependence of PHEMA impulse shear modulus is not described
by the classical Halpin-Tsai equation (HALPIN; KARDOS, 1976) valid for particle composites, but a linear dependence is observed.
These findings confirmed the hybrid character of pHEMA nano-silica
compounds.
On
the other hand, the same
stiffness can be achieved at a much lower volumetric loading
by using the nanodiamond, i.e., between 1 and 5% in the dry state.
Figure 10 shows a schematic
of self-assembled PHEMA
nanodiamonds, while Figure 11 compares the shear modulus
that can be obtained using nanoparticulate amorphous particles and nano-diamond crystalline particles.
The
red points are the
2 and 5% nano-diamond shear modulus, measured in the shear mode using a dynamic
mechanical tester (AVERSA
et al., 2017b).
These preliminary tests have confirmed our estimated theoretical values for hybrid
configurations. Therefore, the adaptive properties of the bones can benefit
from the use of biomechanical and biomechanical materials (biomimetics).
"In vivo" Evaluation of bioactivity and osteoinduction
of implants
The in vivo study
aimed at assessing the bioactivity and osteoinductivity of the ceramic-polymer hybrid scaffold was presented by Aversa et al. (2016d). Unmodified titanium dental implants and siliconized nano-hybrid implants were tested for 2 months at the laboratory hamster femur. Micro-tomography was performed to evaluate
bone density and distribution
around the implant (AVERSA
et al., 2019).
The biomechanical and active osteoinductive characteristic of hybrid materials is summarized in Figure 12, in which microscopic microscopy and bone reconstruction and biomechanical analysis by finite
element analysis are compared
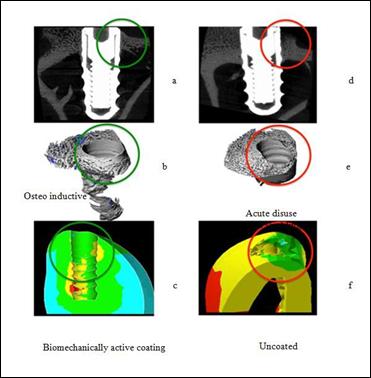
for the same active (left) and non-right biomechanical implants.
Active biomechanical coverage (100 microns) demonstrated that it is able to transfer
the physiological strains
necessary to avoid stress
protection and bone resorption in the vicinity
of the implant
neck (the green cross section of Figures 12a- 12c).
The explant implant (on the right side of Figure 12) shows a significant bone resorption process
in the same area (red circulating in Figures
12d-12f), which was correctly
predicted using the biofilm model (Figure 6b) for the final
bone implant figure 12f ).
FEM
analysis in the same area of the hybrid biomaterial coated implant predicted
a more physiological stress distribution due to the active biomechanical interface that stimulates
osteoblast growth (Figure 12c).
Commercial 2-hydroxyethyl methacrylate was purchased from Sigma-Aldrich Chemicals Co., (St. Louis, MO, USA). Smoke silicon dioxide (Aerosil
300 Degussa, Germany)
with an average
diameter of 7 nm and a specific
surface area of 300 m2 g-1 was used as a bioactive
filler. The initiator, α-α 'azoisobutyronitrile (AIBN), was purchased from Fluka (Milan, Italy). HEMA monomers with an increasing amount of chemical silica (4 to 30% by volume) were mixed. The resin was poured into 2 mm flat plates,
polymerized in an air circulation oven set at 60 °
C for 24 hours and
finally treated at 90 ° C for 1 hour.
Figure
12: Comparison of “in vivo” and Finite Element Biomechanical Analysis results
on a dental implant with and without the biomechanically and osteoinductive hybrid
coating
Planar samples were used for sorption and swelling experiments with aqueous isotonic
saline (0.15 M NaCl). The aqueous solution absorbed in the initially dried samples was determined at equilibrium by gravimetric measurements in a 0.1 mg Mettler Toledo balance sheet (Milano,
Italy). Advanced inflation left in the abnormal sorption
of sample II was monitored
by measuring the time of thickness
of the non- deposited residual glass core. The sorption and balance
swelling experiments were performed at 37 ° C (thermostatic water bath) until the monitoring of the constant weight
absorption monitoring (100 hours) was
monitored.
Solid dental implant models were created using the Solidwork 2007 software. Titanium implant and hybrid material replacing one part were molded. The FE model was obtained by importing
solid models into ANSYS rel. 9.0 FEM software
(Ansys Inc. Houston) using the IGES format.
The volumes were linked to tetrahedral elements, resulting in a 3D FE model consisting of 31,240
of 309 elements and 35,841 nodes. The
precision of the model was
verified by convergence tests.
Measurement of the elastic modulus by sampling the hybrid nanocomposite and the swollen
p-HEMA hybrid
was performed using a mechanical shear
METTLER-TOLEDO (DMA) (Zurich, Switzerland). The elastic
and viscous components of the shear modulus
were measured with a constant
frequency in an isothermal state. The samples were vacuum dried at 60 ° C for 24 hours before
testing. In shear mode, discs of 10 mm diameter
and 2 mm thick discs are placed between
three steel plates forming
a symmetrical sandwich.
An isothermal scan at 37 ° C was carried out in a nitrogen purged medium. The deformation control
was set at 10 μm and a force limit of 0.9 N was applied at an oscillating
frequency of 10
Hz.
The in vitro study aims to evaluate
the potential of nanomaterials to improve
the nanomaterials of nanomaterials Nanomaterials Nanomaterials (AVERSA et al., 2016b; SORRENTINO et al., 2007; SORRENTINO et al., 2009). Both the test body and the control
body were randomly assigned to four groups; each test group consisted of 9 implants,
while each control group consisted
of three fixed elements.
Insertion values ranging between
43.4 and 44.5 and between
44.2 and 45.7 Ncm were recorded in the control and control
group.
In groups 1 through
4 and in groups 5 to 8, the implants
were removed after 1, 6, 12 and 24 hours. The values of the removal torque
were recorded
as described
above. The in vivo study aims at assessing
the bioactivity and osteoinductivity of the ceramic-polymer hybrid scaffold.
Dental prostheses Unmodified titanium and modified
and coated prostheses were implanted into the rabbit
femur and eliminated after two months.
The micro-calculus tomography was performed on the explanted
femur, and the bone density
and distribution on the implant was
evaluated.
Our
research aims at designing a completed
biomimetic dental implant to stimulate
normal OB growth
in adaptable mandibular bones. To achieve
this result, both a suitable biomimetic scheme material and an external
implant screw piece should
be designed.
The biomimetic feature of our hybrid materials has been investigated for both mechanical properties and swelling
properties. The physiological behavior of the bone material
to be imitated from the bioactive material
of the scaffold refers to the following
aspects:
Mechanical properties (dry and swollen) Bioactivity (in vivo implant)
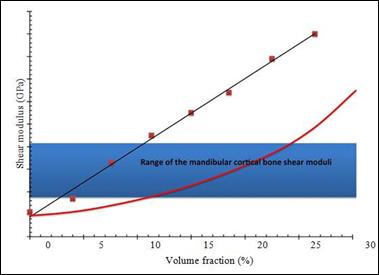
The PhEMA nanocomposite hybrid components with a concentration of 4 to 30 percent silicon dioxide volume were mechanically mechanically mechanically tested isometric, operating in a nitrogen atmosphere at 10 Hz and 37 ° C predominantly viscous viscosity of all compositions. The measured shear modulus is shown in Figure 13. The measured shear modulus of PHEMA-Nanosilica composites does not follow
the classical Halpin-Tsai equation for particulate composites (the ascending
line shown in Figure 13).
A
linear dependence of the shear modulus
values on the progressive loading
of nano silicon was observed.
This unexpected behavior
indicates the hybrid nature of PHEMA nano-silica
composites.
Figure
13: Shear moduli of the hybrid nanocomposites at different nano silica filler loading.
The theoretical Halpin-Tsai curve is reported for comparison in the figure
To
determine the appropriate nanofiller / p-HEMA ratio of the appropriate potential hybrid nanocomposite, the requirements of the target properties
are: Similar to bone rigidity
during implantation.
Shear modulators comparable to those of cortical bone were measured dry for nano silicon fractions
ranging from 4 to 12%. A 5% volume fraction
was then chosen
for sample preparation and FEA simulations (AVERSA
et al., 2016b; AVERSA et al., 2016; AVERSA et al., 2016c; AVERSA et al., 2016d; AVERSA;
APICELLA, 2016).
Elastic modulating (traction test) ranging from 2-20 MPa (strain-curing effect) was measured
for the swollen
hybrid composite (5% by volume).
This value becomes
comparable to that of the tense periodontal ligament
under the same conditions as articular cartilage.
The 5% hybrid nano-composite swells dramatically in isotonic aqueous solution, raising 50% of its dry weight while reducing
the shear modulus
to 2-3 MPa (measured
in DMA at 10 Hz). Such a phenomenon is associated with plasticization of the water-induced polymer that reduces the transition temperature of the polymer
glass below the test temperature 311. The sorption behavior was investigated in a
0.15 M isotonic isotonic isotonic
solution maintained at 37 ° C by hybrids
of glass with
a volume
fraction of 5% both for the weight absorption of the
solution and for the swollen kinetics.
After exposure to the aqueous
solution, 2 mm thick coated PHEMA glass plates begin to swell with a clear face dividing the outer rubber portion and the untreated glass core.
The thickness of the correct glass progressively reduces
the front feed through
the sample. A measure
of the swollen kinetics,
which was reported in Figure
14 based on the square
root of time, is given by the rate of reduction
of the
glass core as a function
of time.
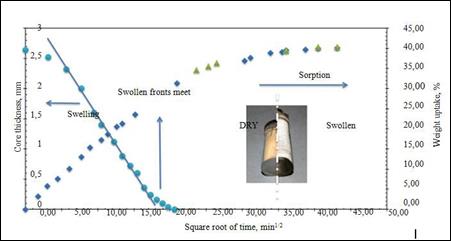
Figure
14: Swelling and sorption kinetics of a 5% by volume hybrid nanocomposite in 0.15M
NaCl water solution (isotonic)
The swollen face has initially advanced
at a constant
rate, according to the relaxed relaxed
relaxed mechanism of anomalies,
indicated as "case II sorption".
An initial
linear inflation rate is approximately 0.10 mm per hour. Since
the swelling
continues, however,
the diffuse resistance develops into the exfoliated outer skin, resulting
in diffusion-controlled swelling
of the remaining
glass core. When inflated fronts meet, the weight gain of the samples is about 27% but continues
to increase to a balance of 40%. This is due to complete balancing through the thickness of the sample.
At steady state, a 14.5% increase
in sample thickness
and a 50% increase in volume were measured.
These values were used to evaluate by simulating the analysis
of finite elements the dimensional changes that appear in the complex geometry of the modified
dental implant described in the following
paragraph.
The use of the active and biocompatible biomechanical interface has been "engineered" to reproduce
bone distribution compatible with the biomimetic. Intervals of physiological strains
and adaptive bone properties are reported.
There are superior surfaces (> 3000 m) (<50
m) that do not favor healthy bone growth.
The variable tension
of adaptive bone growth is bone resorption (<50 m), remodeling
(50- 1500 m), organized
growth (1500-3000 m), resorption (> 3000 m).
Biomimetic aspects are investigated using hybrid
osteoconductive nano- composite coupled with FEM modeling
of swelling and deformation of the hybrid
material. The proposed
solid CAD model of the new ceramic-polymer modified implant
is shown in Figure 15.
Two biomechanical functions were considered during implant design: fixation of the implant
and stimulation of bone growth. A portion of the Ti screw
was replaced by the hybrid nano-composite that maintains the continuity of the outer yarns, while the interior, in which the remaining Ti core is conical through the threaded
tip, has a thickness of between
0.5 and 0.8 mm.
This thickening of the hybrid
ceramic-polymer hybrid nanocomposite produces, following inflation, a progressive volumetric increase
of
the peak. The scaffold
should play two biomechanical functions: one structural as part of the fixation device and one bioactive
as a stimulation stimulus of the bone. From a physiological point of view, stretching the periodontal ligament causes the new bone to fall into the toothbrush. Since the modulus
of
elasticity of the swollen
scaffold was comparable
to that of the periodontal ligament, the analysis of the finite
element confirmed that swelling
of the nanocomposite could act as a
biomechanical entry of bone
growth.

Figure
15: Thick elastic scaffold hybrid material mimicking periodontal ligament
functions in the Biomimetic implant: CAD solid model and a prototype for use in
“in vivo” tests of the new ceramic-polymeric modified Titanium implant
Figure 16 shows the results of a finite element analysis performed on the new implant simulating the swelling of the polymer-ceramic polymer insert in a physiological fluid: displacements up to 0.2 mm were calculated (after 8 hours depending
on the swelling velocity of 0.1 mm / h). This occurrence favors fixation and stabilization of the prosthetic device after implantation (the first biomechanical function).
Moreover, since remodeling is not triggered by the main stress
but by dynamic
loads (not static charges) on remodeling, a positive
growth stimulus is due to the presence of a deformed
hybrid at a physiological compression of 5-30 m; and stress
stress during bone healing and integration of the bone implant (second biomechanical function),
(AVERSA et al., 2016a;
AVERSA et al., 2019).
The blooming of the ceramic-polymer hybrid insert
then sets the implant into the bone and creates an active biomechanical interface for bone augmentation. Bone stress
was modulated by choosing a skeletal
swollen thickness
for healthy bone growth.
The in vivo tests performed using this new modified
oral implant confirmed
the improved ability
of these implants
to promote early osteointegration.
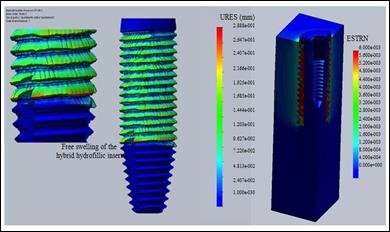
Figure
16: Displacements (URES) in mm and strains (ESTRN) of the hybrid
ceramic-polymeric insert undergoing physiological fluid free and constrained
swelling after implantation
This in vitro study aimed at assessing the possibility of improving
the primary stability of oral implants
through three-dimensional schemes consisting of a hybrid
polymeric material of
nanocomposite ceramic materials.
In
the test groups,
mean withdrawal rates increased progressively over time (diamond points) ranging from 61.2 after 1 hour to 86.2 Ncm after 24 hours, showing
how swelling of the scaffold improved the stability of the primary implant. In contrast,
in control groups (squares), the average values of the removal torque were between
43.7 and 44.9 Ncm.
Figure 17 compares the average
growth of the removal torque and the sorption/inflation kinetics.

Figure
17: Comparison between the kinetics of after implantation removal torque increases
and physiological fluid uptakes in modified implants undergoing swelling
The results of the Micro CT scan are shown in Figure 18. Thin hybrid implants
showed two main differences between osteoinductivity and bioactivity compared to unmodified implants:
•
avoiding bone resorption in the cortical
bone surrounding the implant neck
• improving osteoinduction
in the bone marrow
Immediate titanium implants showed significant bone resorption in the cortical bone.
This effect may be related to the biomechanical stimulation of incorrect cortical
bones outside the range of 50-3000 m. To avoid this undesirable effect due to a proper
mechanical stimulus induced by the interface of the hybrid bioactive hybrid material.
Furthermore, bone growth on the implant surface (the left implant in Figure 18) is due to the osteoinductivity of the hybrid skeleton material tested (AVERSA et al., 2016b; AVERSA et al.,
2016c; AVERSA et al., 2016d).

Figure
18: Biomechanicanics in adaptive morphology of the bone and osteoinduction
in the hybrid scaffolding materials
Biomaterials Today's science is a very interdisciplinary field that plays a central
role in the development of tissue engineering applications involving close collaboration between biologists, chemists, material engineers, physicists and clinicians who have researched in this area at a favorable level to new development systems. Regenerative medicine has developed
a lot and will guarantee many pat- to-bed applications (MONTHEARD et al., 1992; FILMON et al., 2002; DAVIS et al., 1991; KABRA et al., 1991;
APICELLA et al., 1997; PELUSO et al., 1997; CHOW et
al., 2010).
Biomimetic Ability (APICELLA; HOPFENBERG, 1982; APICELLA et al., 2010; ;
APICELLA et al., 2011; APICELLA
et al., 2015; AVERSA et al., 2009; PERILLO
et al., 2010; PETRESCU
et al., 2015; SCHWARTZ-DABNEY; DECHOW, 2003; TÖYRÄSA et al., 2001; FROST, 1990; GRAMANZINI et al., 2016;
HOLLEY et al., 1970; NICOLAIS et al., 1984; AVERSA et al., 2016a).
The new hybrid nanoparticles are prepared
by polymerizing the hydroxy-ethyl- methacrylate monomers filled with detonating nanotubes (up to 5% by volume). This material
absorbs water and swells in aqueous physiological solutions (up to 40-45% by weight),
transforming it into glassy and rubbery conditions. Low levels of non- diamond loads
can improve
the mechanical properties of hybrid materials.
Hydromechanically compatible hybrid hydrogels can be used as scaffolding materials
to increase stress
adaptation mechanisms to
micro and macro
prostheses.
The introduction of active biomechanical interfaces will improve biomimetic implantation while reproducing the biomechanical functions of cartilage and ligaments (APICELLA; HOPFENBERG, 1982; APICELLA
et al., 2010; APICELLA et al., 2011; APICELLA
et al., 2015; AVERSA
et al., 2009; PERILLO
et al., 2015; SCHWARTZ-DABNEY; DECHOW, 2003, TÖYRÄSA et al.,
2001; FROST, 1990; GRAMANZINI et al., 2016; HOLLEY et al.,
1970; NICOLAIS et al., 1984).
The use of metallic microtrabecular prostheses (AVERSA et al., 2016c;
AVERSA et al., 2016d)
coated with ceramic-polymeric hybrid scaffolds (AVERSA et al., 2016d) was proposed to recreate macro and micro-distribution
of stresses
and
deformations in the bone.
The development of polymer hybrid nanocomposites has been proposed in recent studies
(AVERSA et al., 2016b; AVERSA et al., 2016c; AVERSA et al.,
2019). These hybrid materials can induce the mechanical and biological properties needed to
promote healthy local generations.
The innovative features of a biomimetic approach are that the prosthesis is now designed to replace
the joint, damaged by various
causes, but does not stimulate
tissue regeneration. Also, the average length of a prosthesis is about 10/15 years,
while the new "biomimetic
prosthesis" will
last longer, estimated at 20/25 years.
This is very important because the average life span has increased significantly, increasing the number
of orthopedic surgeries
and health and social care
costs.
A biomimetic/biomechanical approach has been developed
in the design of a new modified dental material with bioactive ceramic
hybrid material for biomechanical stimulation and potential
improvement of the mineralization and ossification of the scaffold.
A polymer (pHEMA) filled with silica nanoparticles (4-6% by volume) was chosen as biomimetic material.
This material swells (approximately 14% linear)
in the presence of an aqueous
physiological solution
(when in an aqueous biological environment), raising up to 50- 30% by weight of water (depending on the nano-silicon load) of glass and soft rigid and rubber.
The mechanical behavior of the proposed hybrid materials is comparable to that of the bone when vitreous and cartilage (ligaments) when it is rubber
after swelling.
The bio-imitative properties of this very osteoconductive biomaterial have been used to develop a new bio-dental implant. The new concept is driven by the consideration
that a bioactive
interface
between the
implanted bone and
the prosthetic device is generated
when the material is able to stimulate the implant surrounding the bone in the physiological range of the strain for healthy bone remodeling and organized growth (50-3000).
The use of mechanically-compatible hybrid hydrogels as scaffolding materials is expected to increase
prosthetic adaptation mechanisms that introduce active interfaces that improve the implant
biomimetic while reproducing the biomechanical functions of cartilage and ligaments. The adaptive properties of biomimetic biomaterials (compatible with biomechanics and bioactive) are combined
with new prostheses (AVERSA et al., 2016a;
AVERSA et al., 2016b; AVERSA et al., 2016c; AVERSA et al., 2016d).
5. CONCLUSIONS
It is necessary to develop new biomaterial technologies to produce
scaffolds and bone substitutes that could play a fundamental role in bone regeneration. Bone forms
must exhibit specific
intrinsic characteristics to function as a replacement of the actual bone,
respecting biological, mechanical and geometric constraints.
5.1.
These
features include:
·
Biological Requirements -
Computerized schemes should allow cell adhesion and homogeneous distribution,
growth of regenerative tissue, and ensure the passage of nutrients and chemical
signals. This achievement was achieved by controlling the scaffold porosity;
·
Mechanical requirements -
Schemes must maintain mechanical and hardness properties that allow
osteoblastic colonies to have physiologically and bio-actively controlled deformations.
This was done by correctly modifying the composition of the ceramic-polymer composition
(in our case, 10% by volume amorphous nano-silica).
The combination of clinical observation of traditional implantation behavior will be used to validate
the bio-fidelity of FEM models, while the comparison of in vitro simulated in vitro growth
of osteoblastic colonies
would allow us to explore many new ideas in the design, design, and fabrication of new structure nanostructured cellular
cells with increased functionality and
increased cellular interaction.
This proves to be particularly useful in the direct
design and manufacture of the complex skeletal scaffold.
The new type of biomimetic implants can find applications in the knee, ankle, hip, shoulder and
orthopedic column.
Another area of application of biomimetic schemes is surgical oncology to support and facilitate bone regeneration, resulting in massive
losses due to primitive
and metastatic tumor removal
interventions.
The prosthetic system could allow for better
functional recovery by promoting bone recreation to ensure good maintenance even if it will have an impact on the quality of life of the individual patient severely affected
by the oncological pathology underlying
it.
The biomimetic solution combining a metal support
structure (to guarantee
load resistance) an osteoinductive and biomechanically
active bone (which promotes bone regeneration) finds replication in all surgical treatment
areas involving bone removal
and requires regenerative stimulation of the resected
tissue. In fact, the concentrated bone marrow
contains growth factors and
mesenchymal stem cells that can specialize
in bone cells, cartilage cells, and tendons.
A biomimetic/biomechanical approach has been developed in the design of a new dental material
modified with hybrid ceramic hybrid material for biomechanical stimulation and potential mineralization and ossification of the scaffold.
Polymer (pHEMA) filled with nano silica particles
(4-6% by volume) were chosen as biomimetic material. This material
swells (approximately 14% linear) in the presence of an aqueous
physiological solution
(when in an aqueous biological environment), raising up to 50-30%
by weight of water (depending on the nano- silicon
load) of glass and
soft rigid and rubber.
The mechanical behavior of the proposed hybrid
materials is comparable to that of the bone when
it is glassy and cartilage (ligaments) when it is rubber
after swelling.
The bio-imitative properties of this very osteoconductive biomaterial have been used to
develop a new bioactive
dental implant.
The new concept is driven by the fact that the bioactive
interface of the scaffold between the implanted bone and the prosthetic device is generated
when the material is able to stimulate the implant surrounding the stem to reshape the healthy bone
and increase growth (50-3000).
The use of mechanically-compatible hybrid hydrogels as scaffolding materials is expected to increase
prosthetic adaptation mechanisms that introduce active interfaces that improve the implant
biomimetic while reproducing the biomechanical functions of cartilage and ligaments; (Adaptive properties of biomimetic biomaterials
biomechanically and bioactive compatible of biomass, combined with new prostheses).
6. ACKNOWLEDGEMENT
The Authors acknowledge Liquid Metals Technologies Inc, Ca USA that kindly
supplies the samples for
the characterization.
7. FUNDING INFORMATION
This research has been funded by Italian Ministry of University
and
Research project FIRB Future in Research 2008 project RBFR08T83J.
REFERENCES
ANNUNZIATA, M.;
AVERSA, R.; APICELLA, A.; ANNUNZIATA, A.; APICELLA, D.; BUONAIUTO, C.; GUIDA,
L. (2006) In vitro biological response to a light-cured composite when used for cementation of composite inlays, Dental Materials, v. 22, n. 12, p.
1081-1085. Doi: 10.1016/J.DENTAL.2005.08.009
ANNUNZIATA, M.; GUIDA, L.; PERILLO, L.; AVERSA, R.; PASSARO, I.
(2008) Biological response of human bone marrow stromal cells to sandblasted titanium nitride-coated implant surfaces. J. Mater. Sci. Mater. Med., v. 19, p. 3585-3591. Doi: 10.1007/s10856-008-3514-2.
APICELLA, A.;
CAPPELLO, B.; DEL NOBILE, M. A.; LA ROTONDA, M. I.; MENSITIERI, G. (1993)
Poly(ethylene oxide) (PEO) and different molecular weight PEO’s blends
monolithic devices for drug release. Biomaterials, v. 142, p. 83-90. Doi:
10.1016/0142-9612(93)90215-N
APICELLA, A.;HOPFENBERG,
H. (1982) Water-swelling behavior
of an ethylene-vinyl alcohol copolymer
in the presence of sorbed sodium chloride. J. Applied Polymer
Sci., v. 27, p. 1139-1148. Doi: 10.1002/app.1982.070270404
APICELLA, D.; AVERSA, R.; FERRO, E.; IANNIELLO, D.; APICELLA, A. (2010) The
importance of cortical bone orthotropicity, maximum stiffness direction and thickness on the reliability of mandible
numerical models, Journal of Biomedical Materials Research - Part B Applied
Biomaterials, v. 93, n. 1, April,
p. 150-163,
n. 8: Doi: 10.1002/jbm.b.31569
APICELLA, D.; VELTRI, M.; BALLERI, P.; APICELLA, A.; FERRARI,
M. (2011) Influence of abutment material
on the fracture
strength and failure
modes of abutment-fixture assemblies when loaded in a bio-faithful simulation, Clinical Oral Implants
Research, v. 22, n. 2, February,
p. 182-188: Doi: 10.1111/j.1600-0501.2010.01979.x
APICELLA, D.; AVERSA, R.; TATULLO, M.; SIMEONE, M.; SAYED, S.; MARRELLI, M.; APICELLA, A.; (2015) Direct restoration modalities of fractured
central maxillary incisors: A multi-levels validated finite elements analysis with in vivo strain measurements, Dental Materials, v. 31, n. 12, p. e289-e305, Doi: 10.1016/j.dental.2015.09.016
AVERSA, R.; APICELLA,
D.; PERILLO, L.; SORRENTINO, R.; ZARONE, F.; FERRARI, F.; APICELLA, A. (2009) Non-linear elastic three-dimensional finite element
analysis on the effect of endocrown
material rigidity
on alveolar bone remodeling process.
Dental materials, v. 25, p. 678–690: Doi: 10.1016/j.dental.2008.10.015
AVERSA,
R.;
PETRESCU,
F. I. T.; PETRESCU, R. V.; APICELLA,
A. (2016a) Biomimetic FEA bone modeling
for customized hybrid biological prostheses development. Am. J. Applied Sci., v.13, p. 1060-1067. Doi: 10.3844/ajassp.2016.1060.1067
AVERSA, R.; PARCESEPE, D.; PETRESCU,
R. V.; CHEN, G.; PETRESCU, F. I.
T.; TAMBURRINO, F.; APICELLA, A. (2016b) Glassy Amorphous Metal Injection
Molded Induced Morphological
Defects, Am. J. Applied Sci., v. 13,
n. 12, p. 1476-1482.
AVERSA, R.; PETRESCU, R. V.; PETRESCU, F. I. T.; APICELLA,
A. (2016c) Smart-Factory: Optimization and Process Control
of Composite Centrifuged Pipes, Am. J. Applied
Sci., v. 13, n. 11, p. 1330-1341.
AVERSA, R.; TAMBURRINO, F.; PETRESCU,
R. V.; PETRESCU,
F. I. T.; ARTUR, M.; CHEN, G.; APICELLA, A. (2016d) Biomechanically Inspired Shape Memory Effect
Machines Driven by Muscle like Acting NiTi Alloys,
Am. J. Applied
Sci., v.13, n. 11, p. 1264-1271.
AVERSA, R.; PETRESCU,
R. V.; APICELLA, A.; PETRESCU,
F. I. T. (2017a) Nano- Diamond
Hybrid Materials for Structural Biomedical Application. American Journal of Biochemistry and Biotechnology, v.13, n. 1, p. 34-41. Doi: 10.3844/ajbbsp.2017.34.41
AVERSA, R.; PETRESCU,
R. V.; APICELLA, A.; PETRESCU,
F. I. T. (2017b) Modern Transportation and Photovoltaic Energy for Urban Ecotourism. TRANSYLVANIAN REVIEW OF ADMINISTRATIVE SCIENCES
Special Issue, p. 5-20.
Doi: 10.24193/tras.SI2017.1
AVERSA, R.; PETRESCU, R. V.; APICELLA, A.; PETRESCU,
F. I. T. (2019) A Nanodiamond for Structural Biomimetic Scaffolds. Engineering Review, v. 39, n. 1, p. 81-89. Doi: http://doi.org/10.30765/er.39.1.9
BADZIAG,
P.; VERWOERD, W. S.; ELLIS, W. P.; GREINER,
N. R. (1990) Nanometre-sized diamonds
are more stable than graphite. Nature, v. 343, p. 244-245. Doi:
10.1038/343244a0
BARNARD, A. S.; RUSSO, S. P.; SNOOK, I. K. (2003) Structural
relaxation and relative stability of nanodiamond morphologies. Diamond Relat. Mater., v. 12,
p. 1867-1872. Doi: 10.1016/S0925-9635(03)00275-9
BARNARD, A.
S.; STERNBERG, M. (2007) Crystallinity and surface electrostatics of diamond nanocrystals. J. Mater. Chem., v. 17, p. 4811-4819. Doi: 10.1039/b710189a
BEAUPRE G. S.; HAYES W. C. (1985) Finite Element Analysis of a three dimensional open-celled model for trabecular bone. J. Biomech. Eng.,
v. 107, p. 249-56, PMID: 4046566
BONFIELD, W.; GRYNPAS, M. D.; TULLY, A. E.; BOWMAN, J.; ABRAM, J. (1981) Hydroxyapatite reinforced polyethylene — a mechanically
compatible implant material for bone replacement. Biomaterials, v. 2, p. 185-186. Doi: 10.1016/0142- 9612(81)90050-8
COMERUN, H. U. (1986) Six-year results
with a microporous-coated metal hip prosthesis, Clin. Orthop. 208 81
ČEPELAK I.; DODIG, S.; ČULIĆ, O. (2013) Magnesium-more than
a common cation. Med. Sci., v. 39, p. 47-68.
CHANG, Y. R.; HSU, J. H.; K. CHEN,
K.; FANN, W. (2008) Mass production and dynamic imaging of fluorescent nanodiamonds. Nature Nanotech., v. 3, p. 284-288. Doi: 10.1038/nnano.2008.99
CHEN, Q.; ZHU, C.; THOUAS, G. A. (2012) Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Progress. Biomater., v. 1, p. 1-22. Doi: 10.1186/2194-0517-1-2
CHOW, E. K.; ZHANG, X. Q.; CHEN, M.;
LAM, R.; ROBINSON, E. (2010) Nanodiamond
therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci.
Transl. Med., v. 3,
p. 73ra21-73ra21. Doi: 10.1126/scitranslmed.3001713
CORMACK, A.
N.; TILOCCA, A. (2012) Structure
and biological activity of glasses
and ceramics. Philos. Trans.
Math. Phys. Eng. Sci., v. 370,
p. 1271-1280. Doi: 10.1098/rsta.2011.0371
DAVIS,
P. A.; HUANG,
S.
J.; NICOLAIS,
L.; AMBROSIO,
L.
(1991) Modified PHEMA
Hydrogels. In: Szycher M, editor. High performance biomaterials. Lancaster, PA, USA: Technonic. p. 343–68.
FILMON, R.; GRIZON,
F.; BASLIE, M. F.; CHAPPARD, D. (2002)
Effects of negatively charged groups (carboxymethyl) on the calcification of poly(2-
hydroxyethylmethacrylate). Biomaterials, v. 23, p. 3053–9.
FROST, H. M. (1964) Mathematical elements of lamellar bone remodeling. Springfield: Charles C Thomas. p. 22–25.
FROST, H. M. (1990) Structural adaptations to mechanical usage (SATMU). 2. Redifining Wolff’s law: the
bone remodelling problem. Anat Rec, v. 226, p. 414–22.
FROST, H. M. (2003) update of bone physiology and Wolff’s law for clinicians. Angle Orthod, v. 74, p. 3–15.
FROST, H. M. (1994) Wolff’s law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod, v. 64,
p. 175–88.
GRAMANZINI, M.; GARGIULO, S.; ZARONE,
F.; MEGNA, R.; APICELLA, A.; AVERSA, R.; SALVATORE,
M.; MANCINI, M.; SORRENTINO, R.; BRUNETTI,
A. (2016) Combined microcomputed tomography, biomechanical and histomorphometric analysis of the peri-implant bone: A pilot study in minipig model. Dental Materials, v. 32, n. 6, p.
794-806: Doi: 10.1016/j.dental.2016.03.025
GORUSTOVICH, A. A.; ROETHER,
J. A.; BOCCACCINI, A. R. (2010) Effect
of bioactive glasses on angiogenesis: A review
of in vitro and in vivo evidences. Tissue Eng. Part B Rev., v. 16, p. 199-207. Doi: 10.1089/ten.TEB.2009.0416
GREINER, N. R.; PHILLIPS,
D. S.; JOHNSON, J. D.; VOLK,
F. (1988) Diamonds in detonation soot. Nature, v. 333,
p. 440-442. Doi: 10.1038/333440a0
HALPIN J.
C.; KARDOS J. L. (1976) Halpin-Tsai equations: A review,
Polymer Engineering and Science, v. 16,
n. 5, p. 344-352
HEINEMANN, S.; HEINEMANN, C.; WENISCH,
S.; ALT, V.; WORCH, H. (2013) Calcium
phosphate phases integrated in silica/collagen nanocomposite xerogels enhance the bioactivity and ultimately manipulate the osteoblast/osteoclast ratio in a human co-culture model. Acta Biomaterialia, v. 9,
p. 4878-4888. Doi: 10.1016/j.actbio.2012.10.010
HENCH, L. L.;
POLAK, J. M. (2002) Third-generation
biomedical materials. Science, v. 295,
p. 1014-1017. Doi: 10.1126/science.1067404
HENCH, L. L.; THOMPSON, I. (2010) Twenty-first
century challenges for biomaterials. J. Royal Society
Interface, v. 7, p. S379-S391. Doi: 10.1098/rsif.2010.0151.focus
HENCH, L.
L.; WILSON, J. (1993) An introduction to bioceramics. World Sci.,
v. 1, p. 396-396. Doi:
10.1142/2028
HOLLEY, R. H.; HOPFENBERG, H. B.; V. STANNETT,
V. (1970) Anomalous transport of hydrocarbons in polystyrene. Polymer Eng. Sci., v.10, p. 376-382. Doi: 10.1002/pen.760100612
HOPPE, A.; GÜLDAL, N. S.; BOCCACCINI, A. R. (2011) A review of the biological response to ionic dissolution products from bioactive
glasses and glass-ceramics. Biomaterials, v. 32, p. 2757-2774. DOI: 10.1016/j.biomaterials.2011.01.004
HUISKES, R.; WEINANS,
H.; GROOTENBOER, H. J.; DALSTRA,
M.; FUDULA, B.; SLOOFF, T. J. (1987) Adaptive bone remodeling theory applied
to prosthetic- design analysis. J Biomech, v. 20, p. 1135–1150.
HUTMACHER, D. W.
(2000) Scaffolds
in
tissue engineering
bone and cartilage.
Biomaterials, v. 21, p. 2529-2543. Doi: 10.1016/S0142-9612(00)00121-6
JARRE, G.; LIANG,
Y. J.; BETZ, P.; LANG,
D.; KRUEGER,
A. (2011) Playing
the surface game-Diels-Alder reactions on diamond
nanoparticles. Chem. Commun., v. 47, p. 544-546.
Doi: 10.1039/C0CC02931A
JONES, J. R.; CLARE, A.
G. (2012) Bio-Glasses. An Introduction. 1st Edn., Wiley,
Chichester, ISBN-10:
1118346475, p. 320.
JULIEN,
M.; MAGNE,
D.; MASSON, M.; ROLLI-DERKINDEREN, M.; CHASSANDE,
O. (2007) Phosphate
stimulates matrix Gla protein expression in chondrocytes through the extracellular signal regulated kinase signaling pathway. Endocrinology, v. 148, p. 530-537. Doi:
10.1210/en.2006-0763
KABRA, B.; GEHRKE,
S. H.; HWANG, S. T.; RITSCHEL, W. (1991)
Modification of the dynamic
swelling behaviour of pHEMA. J. Applied Polym. Sci., v.42, p. 2409- 2416. Doi: 10.1002/app.1991.070420906
KARAGEORGIOU, V.; KAPLAN, D. (2005) Porosity of 3D
biomaterial scaffolds and osteogenesis. Biomaterials, v. 26, p. 5474-5491. Doi: 10.1016/j.biomaterials.2005.02.002
KIM, H. W.; KNOWLES, J. C.; KIM, H. E. (2004) Development of hydroxyapatite bone scaffold
for controlled drug release
via poly(ϵ-caprolactone) and hydroxyapatite hybrid coatings. J. Biomed. Mater. Res. Part B: Applied
Biomater., v. 70, n. 240-249. Doi: 10.1002/jbm.b.30038
KRUEGER, A.; STEGK,
J.; LIANG, Y. J.; LU, L.; JARRE, G. B. (2008) Nanodiamond: Simple and efficient functionalization of detonation diamond. E Langmuir, v. 24, p. 4200-4204. Doi:
10.1021/la703482v
KRUEGER, A.;
LIANG,
Y. J.; JARRE, G. B.; STEGK, J. (2006) Surface
functionalisation of detonation diamond suitable for biological applications. J. Mater. Chem., v. 16, p. 2322-2328.
Doi: 10.1039/B601325B
LAI, L.; BARNARD, A. S. (2011a) Modeling
the thermostability of surface functionalisation by oxygen, hydroxyl and water
on nanodiamonds. Nanoscale, v. 3, p. 2566-2575. Doi: 10.1039/c1nr10108k
LAI, L.; BARNARD, A. S. (2011b) Stability of nanodiamond surfaces exposed to N, NH and NH2.
J. Phys. Chem. C,
v. 115, p. 6218-6228. Doi: 10.1021/jp1111026
MANO, J. F.; SOUSA, R. A.; BOESEL,
L. F.; NEVES, N. M.; REIS,
R. L. (2004) Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent
developments. Composi.
Sci. Technol., v. 64,
p. 789-817. Doi: 10.1016/j.compscitech.2003.09.001
MARRELLI, M.; FALISI, G.; APICELLA, A.; APICELLA, D.; AMANTEA, M.(2015) Behaviour of dental pulp stem cells on different
types of innovative mesoporous and nanoporous silicon scaffolds with different functionalizations of the surfaces.
J. Biol. Regulators Homeostatic Agents, v. 9,
p. 991-997. PMID: 26753666
MARTIN, R. B.; BURR,
D. B.; SHARKEY, N. A. (1998) Skeletal Tissue
Mechanics. 1st Edn., Springer,
New
York, ISBN-10: 0387984747, p. 392.
MOHAN,
N.; CHEN, C. S.; HSIEH, H. H.; WU, Y. C.; CHANG, H. C. (2010) In vivo imaging and
toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans.
Nano Lett., v. 10, p. 3692-3699. Doi: 10.1021/nl1021909
MONTHEARD, J. P.; CHATZOPOULOS, M.; CHAPPARD, D. (1992) 2-hydroxyethylmethacrylate HEMA; chemical properties and applications in biomedical fields. J. Macromol.
Sci. Macromol. Rev.,
v. 32, p. 1-34. Doi: 10.1080/15321799208018377
MORALES-HERNANDEZ, D. G.; GENETOS, D.C.; WORKING, D. M.; MURPHY,
K. C.; LEICH, J. K. (2012) Ceramic identity contributes to mechanical
properties and osteoblast behavior on macroporous composite scaffolds. J. Funct. Biomat., v. 23,
p. 382-397. Doi: 10.3390/jfb3020382
MOURIÑO, V.; CATTALINI, J. P.; BOCCACCINI, A. R. (2012) Metallic
ions as therapeutic agents in tissue engineering scaffolds: An overview
of their biological applications and strategies for new developments. J. Royal Society
Interface, v. 9, p. 401-419. Doi:
10.1098/rsif.2011.0611
NICOLAIS, L.; APICELLA, A.; NOTARISTEFANO, C. (1984) Time-temperature
superposition of n-hexane sorption in polystyrene. J. Membrane Sci., v. 18, p. 187-196. Doi: 10.1016/S0376-7388(00)85033-4
OZAWA, M.; INAGUMA, M.; TAKAHASHI, M.; KATAOKA,
F.; RÜGER, A. (2007) Preparation and behavior of brownish,
clear nanodiamond colloids.
Adv. Mater., v. 19, p. 1201-1206. Doi:
10.1002/adma.200601452
OSSWALD,
S.; YUSHIN, G.; MOCHALIN, V.; KUCHEYEV, S. O.; GOGOTSI, Y. (2006) Control of
sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective
oxidation in air. J. Am. Chem. Soc., v. 128, p. 11635-11642. DOI: 10.1021/ja063303n
PARFITT, A.
M. (1983) The Physiological and Clinical
Significance of Bone Histomorphometric Data. In: Bone Histomorphometry: Techniques and Interpretation,
Recker, R.R. (Ed.), CRC
Press, Boca Raton, p. 143-223.
PARFITT, A.
M. (1994) Osteonal and hemi-osteonal remodeling: The spatial and temporal
framework for signal traffic in adult human bone. J. Cell Biochem., v. 55,
p. 273-286. DOI: 10.1002/jcb.240550303
PELUSO, G.; PETILLO, O.; ANDERSON, J. M.; AMBROSIO,
M.; NICOLAIS, L. (1997) The differential effects of poly(2-hydroxyethylmethacrylate) and poly(2- hydroxyethylmethacrylate)/poly(caprolactone) polymers on cell proliferation and collagen synthesis by human lung fibroblasts. J. Biomed.
Mater. Res., v. 34, p. 327- 336. Doi: 10.1002/(SICI)1097-4636(19970305)34:3<327::AID-JBM7>3.0.CO;2-M
PERILLO, L.; SORRENTINO, R.; APICELLA, D.; QUARANTA, A.; GHERLONE, E. D. (2010) Nonlinear visco-elastic finite
element analysis of porcelain veneers: A submodelling approach to strain and stress distributions in adhesive
and resin cement. J. Adhesive
Dentistry, v. 12, p. $03-413.
PETRESCU, F. I. T.; CALAUTIT, K. J. (2016a) About Nano Fusion and Dynamic Fusion,
Am.
J. Applied
Sci., v. 13, n. 3, p. 261-266.
PETRESCU, F. I. T.; CALAUTIT, K. J. (2016b) About the Light Dimensions, Am. J. Applied
Sci., v. 13, n. 3, p. 321-325.
PETRESCU, F. L.; BUZEA, E.; NĂNUŢ, L.; NEACŞA, M.; NAN, C. (2015) The role of antioxidants in slowing aging of skin in a human, Analele Univers.
Craiova Biologie Horticultura Tehn. Prel.
Prod. Agr. Ing. Med., v. 20, p. 567-574.
PETRESCU, F. I. T.; APICELLA, A.; AVERSA, R.; PETRESCU,
R. V.; CALAUTIT, J. K. (2016a) Something about the Mechanical Moment of Inertia, Am. J. Applied
Sci., v. 13, n. 11, p. 1085-1090.
PETRESCU, R. V.; AVERSA, R.; APICELLA, A.; LI, S.; CHEN, G.; PETRESCU,
F. I. T. (2016b)
Something about
Electron Dimension, Am. J. Applied Sci., v. 13, n. 11, p. 1272-1276.
PETRESCU, R. V.; AVERSA, R.; APICELLA, A.; BERTO, F.;
LI, S.; PETRESCU, F. I. T. (2016c) Ecosphere
Protection through
Green Energy, Am. J. Applied Sci.,
v. 13, n. 10, p. 1027-1032.
PETRESCU, F. I. T.; APICELLA, A.; PETRESCU, R. V.; KOZAITIS,
S. P.; BUCINELL, R. B.; AVERSA, R.; ABU-LEBDEH, T. M. (2016d) Environmental Protection through
Nuclear Energy, Am.
J. Applied
Sci., v. 13, n. 9, p. 941-946.
PETRESCU, F. I. T.; PETRESCU, R. V. (2017) The Computer Algorithm
for Machine Equations of Classical Distribution. Journal of Materials and Engineering Structures, v.
4, n. 4, p. 193-209. http://revue.ummto.dz/index.php/JMES/article/view/1590
PETRESCU, F. I. T.; PETRESCU, R.
V. (2018) Inverse Kinematics to a Stewart Platform. Journal of Materials and Engineering
Structures, v. 5, n. 2,
p. 111-122. http://revue.ummto.dz/index.php/JMES/article/view/1623
PETRESCU, F. I. T.; PETRESCU, R.
V. (2019) Nuclear hydrogen structure and dimensions, International Journal
of Hydrogen Energy, v. 44, n. 21, p. 10833-10837.
PETRESCU, F. I. T. (2019) About the
nuclear particles’
structure
and
dimensions. Comp. Part. Mech., v. 6, n. 2, p. 191-194.
PRASHANTHA, K.; VASANTH KUMAR PAI K; SHERIGARA, B. S.; PRASANNAKUMAR,
S. (2001) Interpenetrating polymer networks based on polyol modified castor oil
polyurethane and poly-(2-hydroxyethylmethacrylate): synthesis, chemical, mechanical and thermal properties,
bull. Mater Sci., v. 24,
n. 5, p. 535–548.
REILLY, D. T.; BURNESTAIN A.
H. (1974) The mechanical properties of cortical bone. The J. Of bone and Joint Surgery, v. 56 A, n.
5,
p. 1001-1021
REILLY, D. T.; BURNESTAIN A.
H. (1975) The elastic and ultimate properties of compact bone tissue. J. Biomechanics, v. 8, p.
393-405, Doi:10.1016/0021- 9290(75)90075-5
SCHIRALDI, C.; D’AGOSTINO, A.; OLIVA, A.; FLAMMA, F.; ROSA, A. (2004) Development of
hybridmaterials based on hydroxyethylmethacrylate as supports
for improving cell adhesion
and proliferation. Biomaterials, v. 25, p. 3645-3653. Doi: 10.1016/j.biomaterials.2003.10.059
SCHRAND, A.
M.; JOHNSON, J.; DAI, L.; ŌSAWA, E.
(2009a) Cytotoxicity and Genotoxicity of Carbon Nanomaterials.
In: Safety
of
Nanoparticles:
From Manufacturing to Medical
Applications, Nanostructure Science and Technology. Webster, T.J. (Ed.), ISSN-10:
1571-5744, p. 159-187.
SCHRAND, A. M.; HENS, S. A. C.; SHENDEROVA,
O. A. (2009b) Nanodiamond particles: Properties and perspectives for bioapplications. Crit. Rev. Solid State Mater.
Sci., v. 34, p. 18-74. Doi: 10.1080/10408430902831987
SCHWARTZ-DABNEY,
C. L.; DECHOW, P. C. (2003) Variation in cortical material properties
throughout the human dentate mandible. Am. J. Phys. Anthropol., v. 120, p. 252-277. Doi:
10.1002/ajpa.10121
SHENDEROVA, O.; KOSCHEEV, A.; ZARIPOV,
N.; PETROV,
I.; SKRYABIN, Y. (2011) Surface
chemistry and properties of ozone-purified detonation nanodiamonds.
J. Phys.
Chem. C., v. 115, p. 9827-9837. Doi: 10.1021/jp1102466
SORRENTINO, R.; APICELLA, D.; RICCIO, C.; GHERLONE, E. D.; ZARONE,
F. (2009) Nonlinear visco-elastic finite element
analysis of different
porcelain veneers configuration. J. Biomed. Mater. Res., v. 91, p. 727-736. Doi: 10.1002/jbm.b.31449
SORRENTINO, R.; AVERSA, R.; FERRO, V.; AURIEMMA, T.; ZARONE, F. (2007) Three-dimensional finite element
analysis of strain and stress distributions in endodontically treated maxillary
central incisors restored
with different post, core and crown materials. Dent Mater., v.23,
p. 983-993. Doi: 10.1016/j.dental.2006.08.006
SYED, J.; DHARRAB, A. A. L.; ZAFA, M.
S.; KHAND, E.; AVERSA, R.; PETRESCU, R. V. V.; APICELLA, A.; PETRESCU, F. I. T. (2017) Influence
of Curing Light Type and Staining Medium on the Discoloring Stability of Dental Restorative Composite. American
Journal of Biochemistry and Biotechnology,
v. 13,
n. 1, p. 42-50. Doi: 10.3844/ajbbsp.2017.42.50
TAYLOR, D.; HAZENBERG, J. G.; LEE, T. C. (2007) Living with cracks: Damage and repair in human bone. Nat. Mater., v. 6, p. 263-268. Doi: 10.1038/nmat1866
TÖYRÄSA, J.; LYYRA-LAITINENA, T.; NIINIMÄKIB, M.; LINDGRENC,
R.; NIEMINENB, M. T. (2001) Estimation of the Young's modulus of articular cartilage
using an arthroscopic indentation instrument and ultrasonic measurement of
tissue thickness. J. Biomechan., v.34, p. 251-256. Doi: 10.1016/S0021- 9290(00)00189-5
VIECELLI, J. A.; BASTEA, S.; GLOSLI, J. N.;REE, F. H. (2001) Phase transformations of nanometer size carbon particles
in shocked hydrocarbons and explosives. J. Chem. Phys., v. 115, p. 2730-2736. Doi: 10.1063/1.1386418
WOLFF J. (1892) Das Gesetz der Transformation der Knochen. Berlin: A Hirschwald.
XIAO, J.; OUYANG, G.; LIU, P.; WANG, C. X.; YANG, G. W. (2014)
Reversible Nanodiamond-Carbon Onion Phase Transformations, Nano Letters, ACS publications, p. 3645-3652: Doi: 10.1021/nl5014234
YUAN, Y.; WANG, X.; JIA, G.; LIU, J. H.; WANG, T. (2010)
Pulmonary toxicity and translocation of nanodiamonds in mice. Diamond
Relat. Mater., v. 19, p. 291-299.
Doi: 10.1016/j.diamond.2009.11.022
ZHANG, Q.; MOCHALIN, V. N.; NEITZEL, I.; KNOKE, I. Y.; HAN, J. (2011) Fluorescent PLLA-nanodiamond composites
for bone tissue engineering. Biomaterials, v. 32, p. 87-94. Doi: 10.1016/j.biomaterials.2010.08.090